 |
INTENDED
USE:
The MeDiPro anti-EBV IgA ELISA,
is an enzyme linked immunosorbent
assay intended for the detection
and quantitative determination
of human IgA to the early antigen
and nuclear antigen of the Epstein-Barr
virus contained in human serum.
|
|
|
INTRODUCTION:
Epstein-Barr
virus (EBV), a human herpes
virus, is the cause of a major
etiological factor in a number
of human diseases, including
infectious mononucleosis, Burkitt's
lymphoma and nasopharyngeal
carcinoma.
Nasopharyngeal carcinoma (NPC)
is a malignant tumor which occurs
at high frequency among Chinese
living in Taiwan, Hong Kong,
Singapore, Malaysia and South
China. It has poor prognosis
due to that majority of cases
is at late stage of the disease
and therefore diagnosis of NPC
at early stage would be important
to reduce the mortality.
Seroepidemiological data have
shown that sera from NPC patients
have high titers of EBV specific
antibodies. A rise in titer
of serum IgA to EBV viral capsid
antigen (VCA) and EBV induced
early antigen (EA) in patients
with NPC was first reported
by Henle and Henle (1976). IgA
and IgG to EBV membrane antigen
(MA) have also been detected
in sera from NPC patients (Zhu
et al., 1986). Although IgA
responses to VCA measured in
immunofluorescent assays have
generally been used to screen
for NPC, the studies indicate
that the IgA against EA combined
with EB Nuclear Antigen-1 (EBNA-1)
as measured by ELISA showed
better specificity and sensitivity
than the IgA responses to VCA
as measured by immunofluorescence
(Fones-Tan et al., 1994). A
soluble form of the EBV EA+EBNA-1
is the basis of the serology
assay being described in this
instruction manual.
The MeDiPro anti-EBV IgA
ELISA kit, which is developed
in collaboration with Chang-Gung
University, Taiwan, shows superior
performance for differentiating
NPC patients from normal population.
|
|
 |
PRINCIPLES
OF THE ASSAY:
1. Capture of human IgA to EBV
EA+EBNA-1:
Individuals with reactivated EBV
infections (e.g., NPC patients)
produce antibodies to EBV in their
bodies and have in their circulation,
in particular 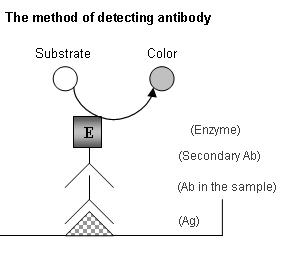 IgA
subclass antibodies to EBV EA+EBNA-1.
Like all serology assays, the
ELISA we have developed is to
quantify the antibodies in serum.
This is accomplished by incubating
dilutions of patient serum in
wells of a microtiter plate which
has the antigen of interest adsorbed
to it, in this case it is EBV
EA+EBNA-1. The antibodies against
EBV EA+EBNA-1 will bind specifically
to the antigens on the microtiter
plate. IgA
subclass antibodies to EBV EA+EBNA-1.
Like all serology assays, the
ELISA we have developed is to
quantify the antibodies in serum.
This is accomplished by incubating
dilutions of patient serum in
wells of a microtiter plate which
has the antigen of interest adsorbed
to it, in this case it is EBV
EA+EBNA-1. The antibodies against
EBV EA+EBNA-1 will bind specifically
to the antigens on the microtiter
plate.
|
|
 |
|
2.
Detection of bound antibodies:
After incubation for the specified
time at the specified temperature,
unbound antibodies are removed
by aspiration and washing. The
presence of bound IgA is then
disclosed by using an anti-human-IgA
antibodies conjugated with of
horse-radish peroxidase (HRP)
and the colorimetric reagent,
TMB. The colorimetric result
can be determined by the microplate
reader. The positivity and the
concentration of antibody of
the unknown samples are then
calculated through an equation
and a standard curve.
KIT CONTENTS:
1. ELISA Plate: One strip holder
containing 8 wells x 12 strips
coated with EBV EA+EBNA-1 antigens.
2. Conjugate: one bottle containing
12 ml of HRP conjugated-goat
anti-human-IgA antibodies.
3. 20x concentrated Washing
Solution: one bottle, 50 ml.
4. Serum Diluents: two bottles,
50 ml each.
5. Positive Control A, 128 EU/ml:
one vial, 800 μl.
6. Positive
Control B, 32 EU/ml: one vial,
800 μl.
7. Positive Control C, 8 EU/ml:
one vial, 800 μl.
8. Negative Control, 2 EU/ml:
one vial, 800 μl.
9. Substrate (TMB): one bottle,
12 ml.
10. Stop Solution (1N HCl):
one bottle, 12 ml.
11. Adhesive plate sealing film:
one sheet.
12. Instruction manual.
|
|
 |
MATERIALS
REQUIRED BUT NOT PROVIDED:
1.1. Micropipettes and tips capable
of accurately delivering from
25 μl to 1000 μl volumes.
2. Multi-channel pipettes and
tips capable of accurately delivering
from 25 μl to 200 μl volumes.
3. 37°C water bath or incubator.
The temperature must be within
37±2°C.
4. Microplate reader. The developed
color should be read on an ELISA
plate reader equipped with a 450
nm filter and a 650 nm reference
filter.
|
|
 |
PRECAUTIONS:
1. Safety considerations
| |
1)
For professionals and in
vitro diagnostic use only.
2) Please refer to the manufacturer's
safety data sheet and the
product labeling for information
on potentially hazardous
components.
3) Human source material
please handle assay specimens,
positive and negative controls
as if they are capable of
transmitting an infectious
agent: Each donor unit used
in the preparation of the
controls was tested by approved
methods for the presence
of antibody to human immunodeficiency
virus (HIV), hepatitis C
virus (HCV) as well as hepatitis
B surface antigen (HBsAg)
and found to be negative.
Because no test method can
offer complete assurance
that HIV, HCV, HBV or other
infectious agents are absent,
these materials should be
handled with good laboratory
practice to avoid skin contact
or ingestion.
4) Do not pipette by mouth.
Avoid contact with skin
and mucous membranes. Avoid
splashing and generating
aerosols.
5) Do not eat, drink, or
smoke in areas in which
specimens or kit reagents
are handled.
6) Wear disposable gloves
throughout the test procedure.
Dispose of gloves in the
biohazard waste. Wash hands
thoroughly afterward.
7) Wipe spills promptly
with 1% sodium hypochlorite
solution (1 to 5 dilution
of liquid household bleach).
Caution: Liquid waste at
acid pH must be neutralized
prior to adding sodium hypochlorite
solutions (bleach) to avoid
formation of poison gas.
Contaminated materials should
be disposed of in the biohazard
waste.
8) Dispose of all specimens
and materials used in the
MeDiPro anti-EBV IgA ELISA
procedure in the biohazard
waste. The recommended method
of disposal is to disinfect
by autoclaving for 1 hour
at 121°C followed by incineration.
Mix liquid wastes with an
equal volume of 5% sodium
hypochloride (liquid household
bleach) and let stand for
60 minutes before disposal.
9) The Controls and 20x
concentrated Washing Solution
contain 0.1% ProClin which
can be absorbed through
the skin and is a sensitizing
agent, please handle carefully.
10) The TMB Substrate Solution
contains tetramethylbenzidine,
hydrogen peroxide and dimethylsulfoxide,
it should be disposed appropriately.
11) The Stop Solution contains
hydrochloric acid. Wear
disposable gloves and protective
glasses when using and disposing
of this reagent.
|
2. Performance considerations
| |
1)
Do not use kit components
beyond the expired date.
Do not mix components
from different lot numbers
except Substrate (TMB) solution,
Stop Solution (1N HCl) and
20x Washing Solution. Serum
Diluents supplied with MeDiPro
anti-EBV IgA ELISA kits
can be used only in
the kits. Do not mix with
components from other manufacturers.
2) Avoid microbial contamination
of reagents. Microbial contamination
may interfere with the sensitivity
of the assay. When not in
use, return all reagents
and kit components to refrigerated
storage (2 to 8° C).
3) Avoid cross-contamination
of reagents. Wash hands
before and after handling
reagents. Cross-contamination
of reagents and/or samples
could cause false results.
Do not interchange vial
or bottle caps and stoppers;
this will lead to cross
contamination of the reagents.
Do not pour reagents
back into vials as reagent
contamination may occur.
4) Shield Substrate (TMB)
solution from light. Aliquot
only the volume of reagents
that is needed. Please do
not use Substrate (TMB)
when blue color occurred.
Do not return used Substrate
to the bottles.
5) To avoid substances which
may interfere with the assay,
use reagent grade quality
water (deionized water that
is bacteria free) to dilute
the 20x concentrated Washing
Solution.
|
|
|
 |
STORAGE
INSTRUCTIONS:
1.
Store MeDiPro anti-EBV IgA
ELISA kits and/or sealed individual
reagents at 2 to 8°C.
2. Opened, unused microplate strips
must be stored at 2 to 8°C in
their original bag with the desiccant
provided.
3. Store diluted 1x Wash Solution
at room temperature (21 to 25°C)
for up to 2 weeks.
4. Avoid storing reagents and
specimens in auto-defrost refrigerators.
|
|
 |
PREPARATION
OF REAGENTS:
1. All the reagents and serum
samples should be brought to
room temperature (21-25°C)
and mix thoroughly before assay.
Do not use reagents beyond the
stated expiration date marked
on the package label.
2. Washing Solution: dilute 1
volume of 20x concentrated Washing
Solution with 19 volume of reagent
grade water. If the kit is used
for multiple times, please prepare
appropriate volume for each time.
3. All serum samples should
be vortexed before use.
4. Dilution of serum samples:
dilute serum samples with Serum
Diluents provided in this kit
as follows:
10 μl of serum + 1 ml of Serum
Diluents
For manual dilutions it is suggested
to dispense the Serum Diluents
into the test tube first and then
add the patient serum. Controls
don't need to be diluted.
|
|
 |
PROCEDURE:
1. Take enough strips and set
on strip holder.
2. Add 100 μl of Serum Diluents
(blank), Negative Control and
Positive Controls (A, B, C) and
diluted serum samples to the individual
well. Duplication is recommended
for each control and blank.
3. Cover the plate with adhesive
plate sealing film. Incubate at
37°C for 60 min.
4. Aspirate or shake out liquid
from all wells. Add 280-300 μl
of diluted 1x Washing Solution.
Aspirate or shake out and turn
plate upside down and blot on
paper toweling to remove all liquid.
Repeat the wash procedure 2 times
(for a total of 3 washes). Repeat
the wash procedure 4 times (for
a total of 5 washes) on automated
equipment.
| **IMPORTANT
NOTE: Regarding steps 4
and 7 - Insufficient or
excessive washing will result
in assay variation and will
affect validity of results.
Therefore, for best results
the use of semi-automated
or automated equipment set
to deliver a volume to completely
fill each well (280-300
μl) is recommended. A total
of 5 washes may be necessary
with automated equipment.
Complete removal of the
washing solution after the
last wash is critical for
the accurate performance
of the test. Also, visually
inspect to ensure that no
bubbles are remaining in
the wells. |
5. Add 100 μl of Conjugate to
each well.
6. Cover the plate with adhesive
plate sealing film. Incubate at
37°C for 30 min.
7. Aspirate or shake out liquid
from all wells. Wash with diluted
1x Washing Solution 3 times. (5
times for semi-automated or automated
equipment)
8. Add 100 μl of Substrate (TMB)
to each well.
9. Cover the plate with adhesive
plate sealing film. Incubate at
37°C for 10 min.
10. Add 100 μl of Stop Solution
(1N HCl) to each well to stop
the reaction. Tap the plate gently
along the outsides to mix contents
of the wells thoroughly.
11. Determine absorbance by reading
assay plate at 450 nm using 650
nm as reference. The plate may
be held up to 30 min after addition
of the Stop Solution before reading.
|
|
 |
INTERNAL
QUALITY CONTROL:
Results of an assay run are valid
if the following criteria for
the Controls are met:
1.
Average blank (buffer only)
well OD450nm-650nm value
< 0.05
2. Average OD450nm-650nm
value of control sera:
Positive Control A: >
1.50
Positive Control C: >
0.30
Negative Control: <
0.2 |
If any value is out of the range
of above criteria, the assay need
repeat.
|
|
 |
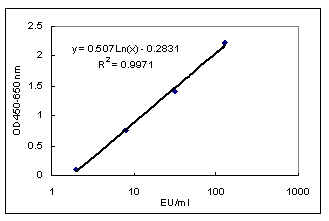
RESULTS:
1. Calculate the EU:
Draw a standard curve on a semilog
paper or computer analysis software
(Microsoft Excel, Lotus123, etc.)
by using OD450nm-650nm of positive
control (A, B, C) and negative
control and corresponding EU/ml
(128, 32, 8, 2 EU/ml). The EU/ml
of each positive serum sample
can be derived from the equation:
Y (OD450nm-650nm) =
a × Ln(X) + b.
For example:
| |
EU/ml
|
OD450nm-650nm
|
| Positive
Control A |
128
|
2.223
|
| Positive
Control B |
32
|
1.410
|
| Positive
Control C |
8
|
0.762
|
| Negative
Control |
2
|
0.096
|
|
2. Cut-off
value (C.O.V.):
C.O.V. = Calculated OD value of
8 EU/ml
For example:
Calculated OD value of 8 EU/ml
= 0.771
|
|
OD Value
|
|
C.O.V.-10%
|
0.694
|
|
C.O.V.
(8 EU/ml)
|
0.771
|
|
C.O.V.+10%
|
0.848
|
The concentration
(EU/ml) can be calculated by
the equation of standard curve.
If the OD450nm-650nm
value of serum sample is higher
than 0.848, the result of this
sample is positive, for confirmation
of NPC, further examination
on clinical symptoms is necessary;
while the OD450nm-650nm
value is lower than 0.694, the
sample is negative. If OD450nm-650nm
value of serum sample is between
0.694~0.848 (C.O.V.±10%), repeat
testing is recommended.
|
|
 |
LIMITATIONS
OF USE:
1. The user of this kit is advised
to carefully read and understand
the package insert. Strict adherence
to the protocol is necessary to
obtain reliable test results.
In particular, correct sample
and reagent pipetting, along with
careful washing and timing of
the incubation steps are essential
for accurate results.
2. This product is specifically
designs for differentiating nasopharyngeal
carcinoma patients from normal
population. The performance characteristics
have not been established for
patients with Burkitt's lymphoma,
other EBV associated lymphadenopathies,
and other EBV associated diseases
other than EBV related mononucleosis.
3. The results of ELISA immunoassays
performed on serum from immunosupressed
patients must be interpreted with
caution.
4. Samples that remain equivocal
after repeat testing should be
retested by an alternate method,
e.g., immunofluorescence assay
(IFA). If results remain equivocal
upon further testing, an additional
sample should be taken.
5. Results of this test should
be interpreted by the physician
in the light of other clinical
findings and diagnostic procedures.
6. Heterotypic (false positive)
IgA responses to EBV may occur
in patients infected with CMV
and also in patients infected
with HSV-1.
7. Specific IgG may compete with
the IgA for sites and may result
in a false negative. Conversely,
rheumatoid factor in the presence
of specific IgG may result in
a false positive reaction.
8. Some antinuclear antibodies
have been found to cause a false
positive reaction on some ELISA
tests.
9. Results from children should
be reviewed with caution.
10. Icteric, lipemic, hemolyzed,
or heat inactivated sera may cause
erroneous results and should be
avoided.
11. Kit procedures or practices
outside those in this package
insert may yield questionable
results.
12. The performance characteristics
have not been established for
any matrices other than sera.
13. The performance characteristics
for this assay have not been established
for pediatric specimens. |
|
 |
|
BIBLIOGRAPHY:
1. Henle G. and Henle W. Epstein-Barr
virus-specific IgA serum antibodies
as an outstanding feature of
nasopharyngeal carcinoma. Int.
J. Cancer 17:1-7 (1976).
2. Zeng Y., Pi G.H., Deng H.,
Zhang, J.M., Wang P.C., Wolf
H.De The G. Epstein-Barr virus
seroepidemiology in China. AIDS
Res. Hum. Retroviruses 2, Supplement
1:S7-S15 (1986).
3. Motz M., Fan J., Seibl R.,
Jilg W., Wolf H. Express of
the Epstein-Barr virus 138-kDa
early protein in Escherichia
coli for the use as antigen
in diagnostic tests. Gene 42:303-312
(1986).
4. Zong Y.S., Sham J.S.T., Ng
M.H., Ou X.T., Guo Y.Q., Zheng
S.A., Liang J.S., Qiu H. Immunoglobulin
an against viral capsid antigen
of Epstein-Barr virus and indirect
mirror examination of the nasopharynx
in the detection of asymptomatic
nasopharyngeal carcinoma. Cancer
69:3-7 (1992).
5. Tatsuya T. Purification and
characterization of the DNA-binding
activity of the Epstein-Barr
virus DNA polymerase accessory
protein BMRF1 gene products,
as expressed I insect cells
by using the baculovirus system.
J. Virology 67:1681-1687 (1993).
6. Zhu XX, Zeng Y, Wolf H. Detection
of IgG and IgA antibodies to
Epstein-Barr virus membrane
antigen in sera from patients
with nasopharyngeal carcinoma
and from normal individuals.
Int. J. Cancer 37:689-691 (1986)
7. Chen MR, Liu MY, Hsu SM,
Fong CC, Chen CJ, Chen IH, Hsu
MM, Yang CS, Chen JY. Use of
bacterially expressed EBNA-1
protein cloned from a nasopharyngeal
carcinoma (NPC) biopsy as a
screening test for NPC patients.
J. Med. Virol. 64:51-57 (2001)
8. Chow KC, Ma J, Lin LS, Chi
KH, Yen SH, Liu SM, Liu WT,
Chen WK, Chang TH, Chen KY.
Serum responses to the combination
of Epstein-Barr virus antigens
from both latent and acute phases
in nasopharyngeal carcinoma:
complementary test of EBNA-1
with EA-D. Cancer Epidemiol
Biomarkers Prev. 6:363-368 (1997)
9. Fones-Tan A, Chan SH, Tsao
SY, Gan LH, Tan WH, Li B, Khong
PW, Gan YY. Enzyme-linked immunosorbent
assay (ELISA) for IgA and IgG
antibodies to Epstein-Barr-virus
ribonucleotide reductase in
patients with nasopharyngeal
carcinoma. Int J Cancer. 59:739-742
(1994)
|
|
|